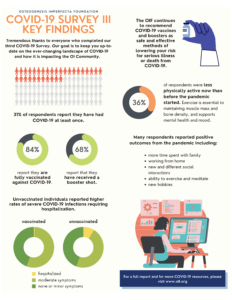
Thank you to all of the 577 OI community members who took the time to fill out the OI Foundation’s third COVID-19 survey! Our goal in gathering the community’s input is to provide feedback during the changing landscape of COVID-19 and direct the OIF’s resources where most needed. CLICK HERE to view the full survey results and important findings.
To complete future surveys about OI, we highly encourage you to sign up for the OI Registry at www.oif.org/OIregistry to be the first to hear about these opportunities.














