National Osteogenesis Imperfecta Awareness Week is May 6 – 13, 2023!
One of the most effective (and free!) ways to raise awareness during National OI Awareness Week (May 6-13, 2023) is through social media. Please join us in bringing attention to this important week by sharing OIF posts, facts about OI, your OI story, and information about OIF programs and events on your social media pages including Facebook, Instagram, Twitter, LinkedIn, and YouTube.
We have provided sample posts for your use below. Stay connected with the OI Foundation and OI community by using the hashtags #OIawarenessweek and #UnbreakableSpirit in your posts.
Sample Posts:
Guidelines for Use: OI Awareness Week logos and graphics may be used for educational and informational purposes in relation to OI Awareness Week. These graphics may not be used to endorse products/services or to solicit funds.
Click here to download these graphics from Dropbox.
National OI Awareness Week
These graphics are perfect to remind your friends and family that a very special week is coming up!
Sample posts:
- National OI Awareness Week is May 6-13! Please join me in raising awareness and support for a community that is so special to me! #OIawarenessweek #UnbreakableSpirit
- I can’t wait for #OIawarenessweek! I will be raising OI awareness by…. I hope you will join me by….
- National OI Awareness Week begins tomorrow! I will be raising OI awareness and hope you will do the same! To raise OI awareness this week, I will… [YOUR RESPONSE HERE]. This is important to me because … For more information on how you can raise awareness and support the OIF this week, visit www.oif.org/awarenessweek. #OIawarenessweek #UnbreakableSpirit
Facts about OI
OI is a rare disease that your social media friends may not know much about, or even heard of! OI Awareness Week is the perfect time to share facts about OI!


Sample posts:
- Do you know what osteogenesis imperfecta (OI) is? OI is a rare and complex genetic disorder of the collagen that is often characterized by bones that break easily. While the major feature of OI is bones that break easily, many other body systems may be affected and individuals with OI may experience dental issues, hearing loss, muscle weakness, cardiac and respiratory issues. OI is highly variable, ranging from few symptoms and fractures to a form that is lethal at birth. Learn more about OI at www.oif.org. #OIawarenessweek #UnbreakableSpirit
- What causes OI? OI is caused by a mutation (change) in collagen, the major protein present in bone. These mutations affect bone composition, formation, and strength, as well as the structure of other tissues. Learn more at www.oif.org #OIawarenessweek #UnbreakableSpirit
Learn More!
Share resources on your social media pages for your friends, family, and followers to learn more about OI.
Sample posts:
- The OI Foundation’s goal is to provide information for OI community members, medical professionals, and caregivers. The OIF’s online Information Center includes medically verified information about OI, OI publications, video resources, and fact sheets. Learn more about OI and the programs of the OI Foundation at www.oif.org. #OIawarenessweek #SHAREforAWARENESS
- Raising awareness of OI is so important, especially during National OI Awareness Week! One of the best ways to understand is to ask questions! Do you have questions about OI? I’ll do my best to answer them and share my experiences! #OIawarenessweek #UnbreakableSpirit
Share Your Story
During OI Awareness Week, your friends and family want to hear from YOU! One of the best ways to raise OI awareness among your friends, family, and community is to share your OI story!
Share your relationship and experience with OI, what having OI means to you, or a personal story.
 How to Support the OI Community
How to Support the OI Community
Your friends, family, and fellow OI community members may want to support National OI Awareness Week, but are not sure where to begin! Share this list of 5 simple ways to participate in this special week!
Sample post:
- There are so many ways to get involved in National OI Awareness Week! It would mean the world to me if my family, friends, and followers would join me in raising awareness for OI this week. For more information about how you can get involved, please visit www.oif.org/awarenessweek. #OIawarenessweek
Click here to download these graphics from Dropbox.
Connect with the OI Foundation on Social Media! Like, follow, share!















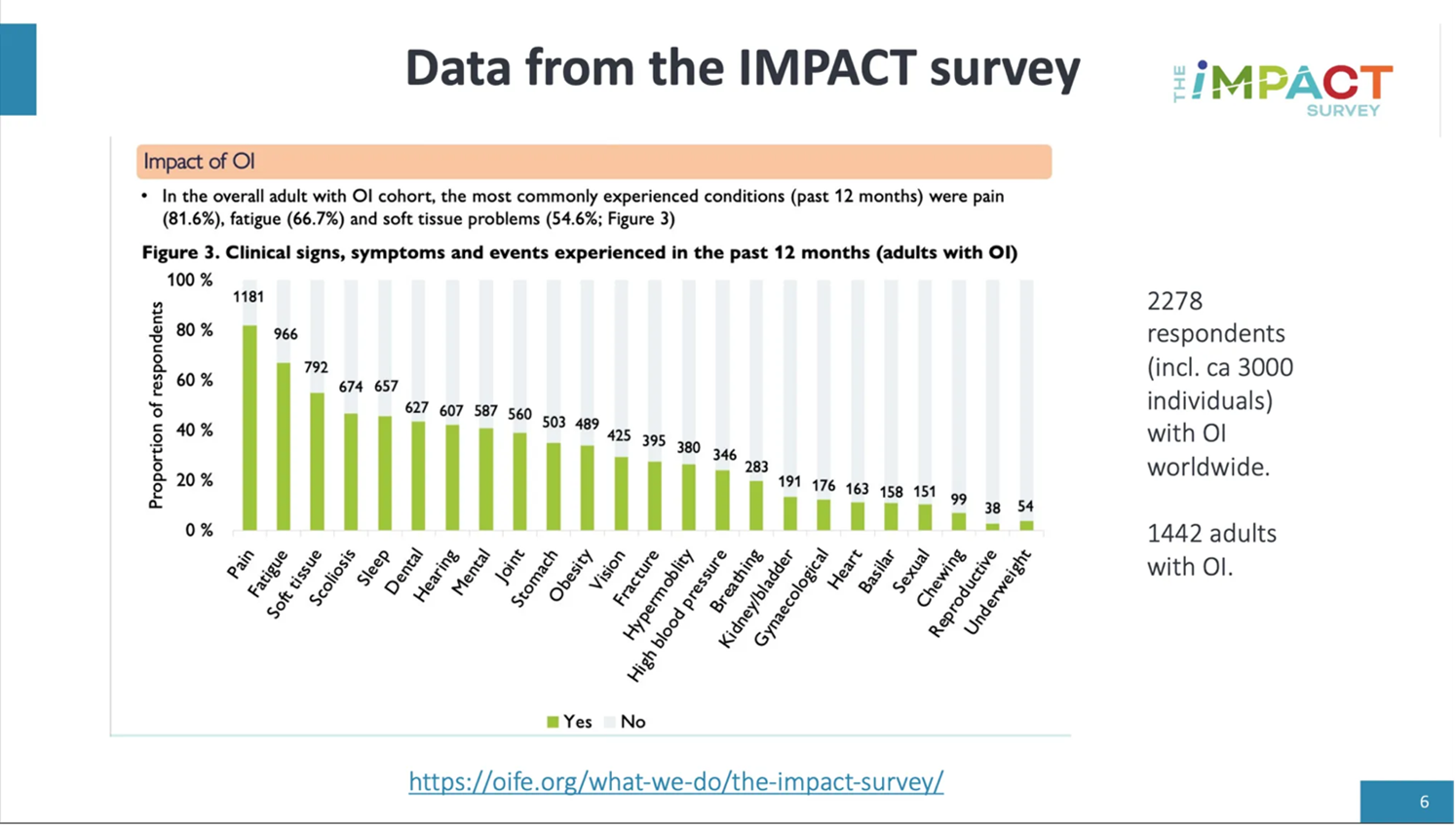

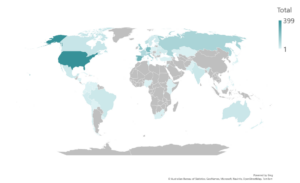
 What is the IMPACT survey?
What is the IMPACT survey?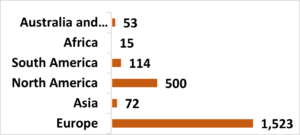




 The February E-Newsletter is here! Check out this issue to learn about grants for OI community members, the Pain and OI Survey, and upcoming OIF programs and events!
The February E-Newsletter is here! Check out this issue to learn about grants for OI community members, the Pain and OI Survey, and upcoming OIF programs and events!