Research
OI Research Update from Ultragenyx
Ultragenyx Pharmaceutical Inc. and Mereo BioPharma Group today announced data from the dose-selection Phase 2 portion of the Phase 2/3 Orbit study, showing that setrusumab significantly increased bone production in…
Read MoreOI Studies (Currently Recruiting)
Participate in OI Research! As you know, supporting research is an important part of the OI Foundation’s mission and we need volunteers to help advance the scientific understanding of OI…
Read MoreOIF Scientific Meeting Recap
Each year during the OI Foundation’s annual Scientific Meeting, leading scientists and medical professionals gather to share their research on osteogenesis imperfecta (OI). Dr. Frank Rauch (OIF Medical Advisory Council…
Read MoreCurrently Recruiting!
7707: Use of clear aligners for the treatment of dental malocclusion in individuals with Osteogenesis Imperfecta Types III and IV Misalignment of teeth may interfere with oral hygiene, gum health,…
Read MoreThe IMPACT Survey
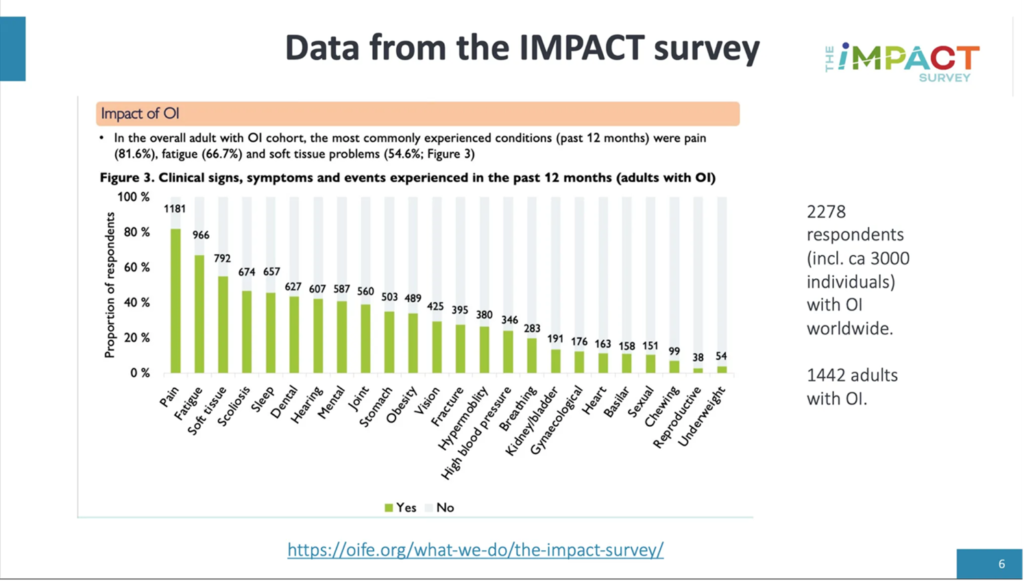
Living With Osteogenesis IMPerfecta: UnderstAnding Experiences Based On Community InsighT and Evidence Survey, the IMPACT Survey What is the IMPACT survey? In 2021, the Osteogenesis Imperfecta Foundation (OIF), the Osteogenesis…
Read MoreUsing the ECHO Model® to Expand Professional Learning Opportunities for Osteogenesis Imperfecta
OIF Director of Education, Michael Stewart is presenting a poster at #RareDiseaseDay at NIH today. Using the ECHO Model® to Expand Professional Learning Opportunities for Osteogenesis Imperfecta From October 2020…
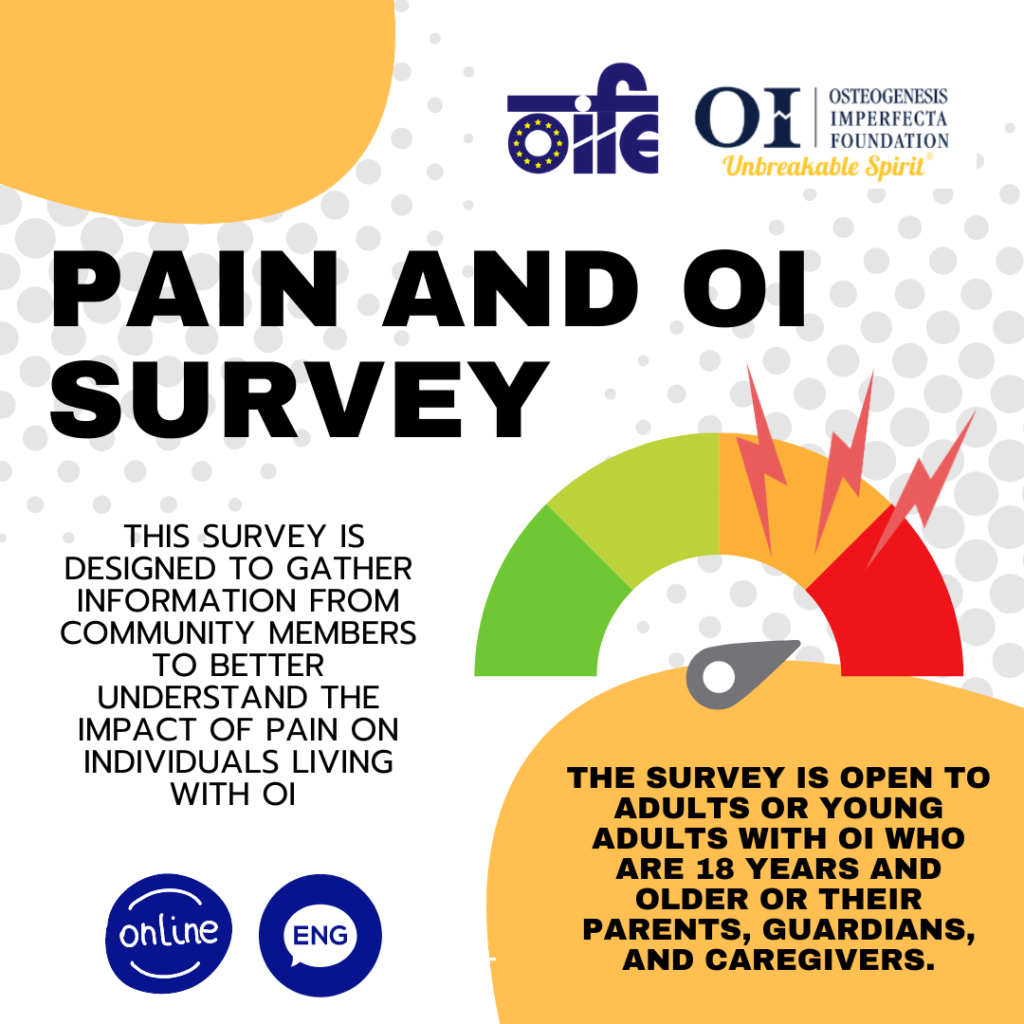
Read MoreComplete the new OI and Pain Survey!
The OI Foundation and OI Federation of Europe (OIFE) have partnered on a new project investigating the experience of pain for people with OI. The following survey investigates the experience of…
Read MoreStudy Announcement: Poise 1
The OI Foundation would like to bring your attention to a phase 1 study of a new medical treatment being conducted by Sanofi. Sanofi is seeking participants from the OI community. Please…
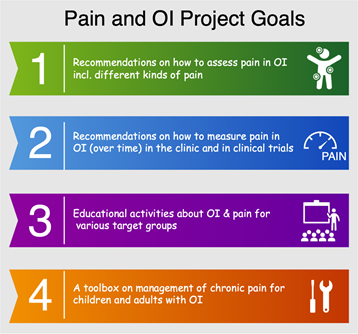
Read MorePain and OI Project
A number of recent surveys have shown that approximately 60% of individuals with OI report that they suffer from chronic pain. To increase research around pain management, the OI Foundation…
Read MoreOI Research Announcement: Use of clear aligners…
The OI Foundation would like to bring your attention to a research study being conducted by the Brittle Bone Disorders Consortium: Please call/email to see if you are eligible to…
Read More