Research
Ultragenyx Receives Breakthrough Therapy Designation for Setrusumab (UX143) in OI
The OI Foundation would like to bring your attention to an update from Ultragenyx! Ultragenyx Receives Breakthrough Therapy Designation for Setrusumab (UX143) in Osteogenesis Imperfecta CLICK HERE TO VIEW THE…
Read MoreOI Research Grants
Michael Geisman Fellowship Grant The Michael Geisman Fellowship Grant program awards funding to post-doctoral trainees who are currently working on projects with clear relevance to osteogenesis imperfecta (OI), or who…
Read MoreOI Research Update: Ultragenyx Orbit and Cosmic Phase 3 Study Enrollment is Complete
We are pleased to share exciting news from our partners at Ultragenyx. They have completed enrollment for their Phase 2/3 Orbit and Phase 3 Cosmic studies. These studies evaluate setrusumab…
Read MoreSomewhere To Go
In October 2023, the OI Foundation hosted a conference titled, Somewhere to Go for Adults with Childhood-Onset Rare Diseases: A Conversation About How We Can Fill Gaps in Care, to…
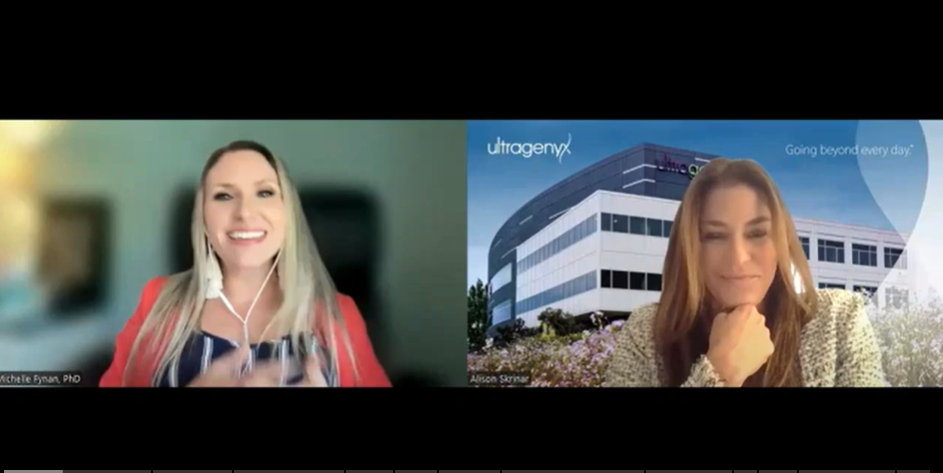
Read MoreUpdate from Dr. Alison Skrinar on the Ultragenyx trials, Orbit and Cosmic
The OI Foundation recently shared an announcement from Ultragenyx which shared Interim data from the Phase 2 portion of their Orbit study. This data showed that treatment with setrusumab reduced…
Read MoreParticipate in the OI Wellbeing Study
Participate in the OI Wellbeing Study Baylor College of Medicine is conducting this OI Wellbeing Study as a part of the Brittle Bone Disorders Consortium which aims to improve the…
Read MoreOI Study: Evidera
Evidera, a company specialized in patient-centered outcomes research, are currently looking for individuals living with osteogenesis imperfecta (OI). Please take a moment to read a message from Evidera below: We…
Read MoreExciting new data update from Ultragenyx!
Today, Ultragenyx announced Interim data from the Phase 2 portion of the Orbit study, which showed that treatment with setrusumab reduced incidence of fractures in patients with OI by 67%…
Read MoreWhat We’re Learning from a Natural History Study
From the BBDC: Osteogenesis Imperfecta Over Time: What We’re Learning from a Natural History Study To learn more about the natural history of individuals with osteogenesis imperfecta (OI), the Brittle…
Read MoreOI Research Update: Abnormal lung function in OI
OI Research Update: Abnormal lung function in osteogenesis imperfecta due to both intrinsic and extrinsic causes Gochuico et al. (2023) J Med Genet May 16:jmg-2022-109009. Doi:10.1136/jmg-2022-109009 Lung disease is the…
Read More