Category: Research
Currently Recruiting!
7707: Use of clear aligners for the treatment of dental malocclusion in individuals with Osteogenesis Imperfecta Types III and IV
Misalignment of teeth may interfere with oral hygiene, gum health, jaw function, opening of the jaw, chewing, breathing, and speech. It can also affect the shape of your face, mouth and overall quality of life. There is very little information on the appropriate treatment for teeth misalignment in OI.
The purpose of this study is to determine if it is safe to use Invisalign clear aligners in correcting the misalignment of teeth in people with OI. Clear aligners are transparent plastic trays that are designed to fit over your teeth. With each new tray, teeth are moved a little at a time until they reach the desired position. We plan to have approximately 57 people take part in this study. If you take part in this study, you will be asked to come to a participating site for up to a total of 15 study visits over a course of 2 years and 4 months (or 28 months).
You may be eligible to participate if you:
- are between ages 12 – 40
- have OI type III or IV
- have had no prior orthodontic treatment
How to participate:
In order to participate, you must contact the study coordinator of any of the participating institutions by phone or by e-mail. Please use the information below to inquire about participation:
National Institutes of Dental Craniofacial Research (Bethesda, Maryland)
Contact: Danielle Elangue MHA, MBA, RN, Research Nurse Specialist ( C )
301-451-9733 | danielle.elangue@nih.gov
University of California at Los Angeles (Los Angeles, California)
Contact: Sarah Gaunt, Clinical Research Coordinator
310 -206 -7176 | sgaunt@mednet.ucla.edu
Disclaimer: The OI Foundation is not involved in the design or management of this research, and as such, is neither endorsing nor supporting this study. The mission of the OIF is to keep the OI community informed of all relevant studies. This information is made available as a service to the OI community. We are available to answer questions on this or any other research announcement. Please contact the OI Foundation at (301) 947-0083 or bonelink@oif.org if you have any questions.
The IMPACT Survey
Living With Osteogenesis IMPerfecta: UnderstAnding Experiences Based On Community InsighT and Evidence Survey, the IMPACT Survey
 What is the IMPACT survey?
What is the IMPACT survey?
In 2021, the Osteogenesis Imperfecta Foundation (OIF), the Osteogenesis Imperfecta Federation Europe (OIFE), and Mereo BioPharma collaborated closely to launch the IMPACT Survey. The goal of this project was to capture and quantify the real impact OI has on the lives of people with OI, their families, and caregivers. We were thrilled that more than 2,200 OI community members from 66 countries participated in this survey!
The survey was based on evidence gaps around:
- Clinical impact of OI on affected individuals
- Humanistic impact on individuals, their families and caregivers
- Economic impact on individuals and wider society
The IMPACT survey is now closed. Thank you to all participants who made this possible.
What were the target groups?
The survey was developed for the following primary target groups:
- Adults with OI (over 17 years old)
- Parents (without OI) of children with OI
- Parents (with OI) of children with OI
- Adolescents with OI (12 – 17 years old)
In addition to the target groups above, there were also a substantial number of responses from
- Parents of adults with OI
- Close relatives of people with OI
There were different questions based on which target group you belong to. Adults with OI who had children with OI, could answer both on behalf of themselves and their children.
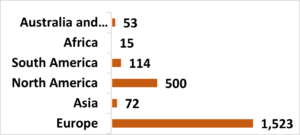
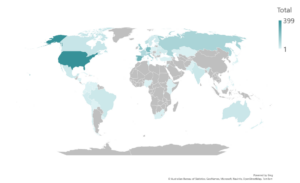
Geographic distribution of respondents
The collaborative survey development allowed members across OI communities and geographies to participate.
What will the data be used for and how will it be stored?
The IMPACT Survey provides a large global dataset describing the experiences of the OI community. The results of this survey are being used to improve healthcare services and potential future treatments for OI.
The data was gathered on a confidential and anonymous basis, with Non-Personally Identifiable Information (Non-PII) . It will be securely stored by Wickenstones according to Data Protection Regulations. The data is now being analyzed according to the plan developed by the Steering Committee, to prepare four proposed central publications on the impact of OI in peer-reviewed, scientific journals.
What data has been published? What are the findings?
So far, the first article “The patient clinical journey and socioeconomic impact of osteogenesis imperfecta: a systematic scoping review” was published in Orphanet Journal of Rare Diseases in February 2023. The purpose of this review was to capture and quantify the published evidence relating specifically to the clinical, humanistic, and economic impact of OI on individuals, their families, and society.
The review suggests that there is limited data regarding health concerns beyond bone health and how these concerns may impact health-related quality of life, in particular that of adult men and other family members.
The four remaining articles will be published in 2023 and 2024.
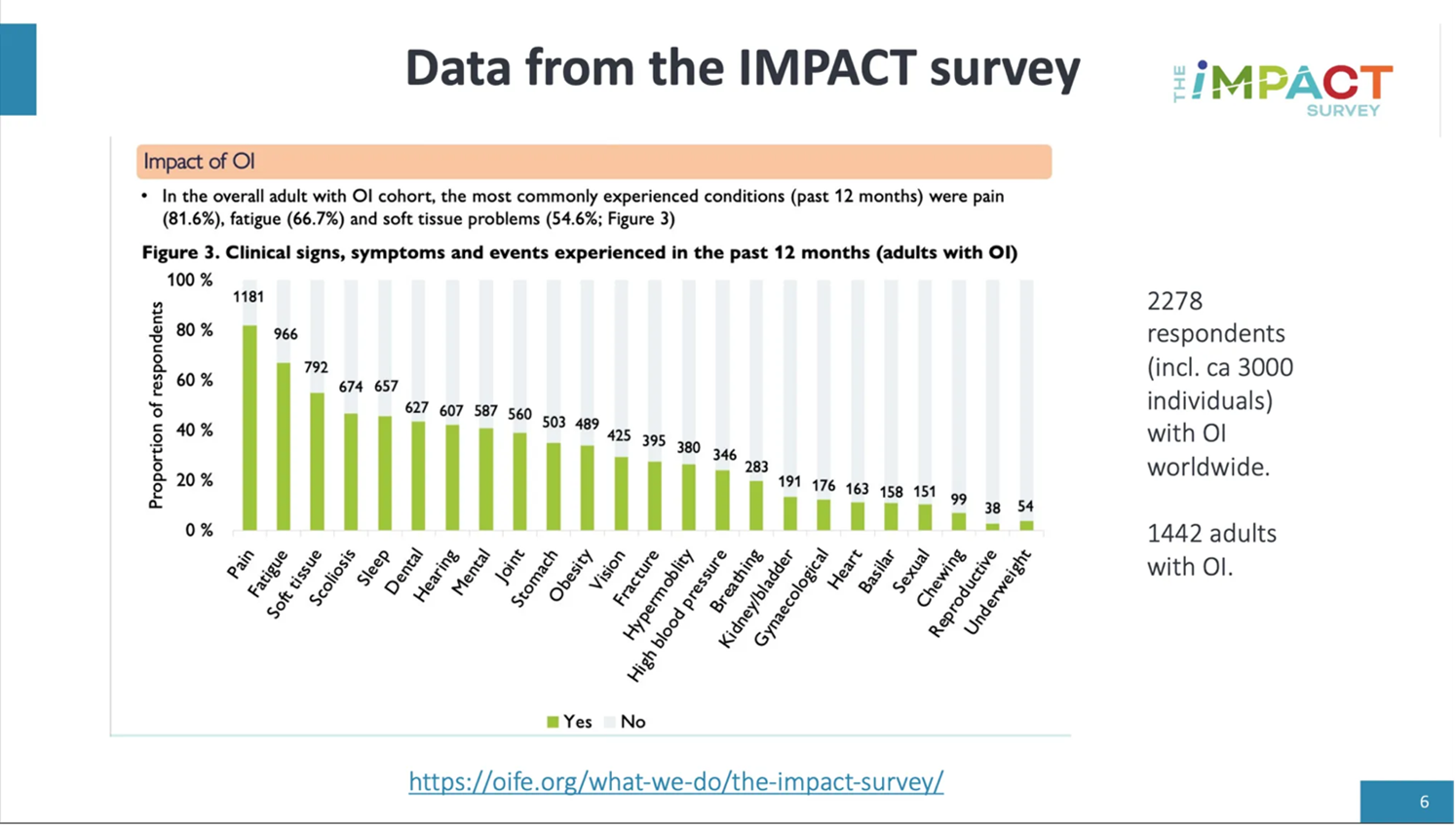
The graphic below describes the most common health conditions in adults with OI (of the past 12-months prior to answering). Pain, fatigue and soft tissue are the most common symptoms, while fractures are in 13th place for the adult population.

We have been given permission to share this graphic.
Using the ECHO Model® to Expand Professional Learning Opportunities for Osteogenesis Imperfecta
From October 2020 to September 2021, the Osteogenesis Imperfecta (OI) Foundation ran a monthly OI TeleECHO Clinic Series program. In every session, expert medical faculty and guest speakers deliver a didactic presentation on diagnosing or treating OI, group discussions, and then followed by participant-led case presentations. These sessions reached hundreds of medical professionals and expanded access to expert medical knowledge on this rare bone disease.
Click here to view the poster.
Complete the new OI and Pain Survey!
The OI Foundation and OI Federation of Europe (OIFE) have partnered on a new project investigating the experience of pain for people with OI. The following survey investigates the experience of pain for people with OI. This survey is for anyone 18 years old or older and was written with input from OI experts from North America and Europe.
Through this survey, the international Pain and OI Project hopes to learn more about the different types of pain people experience, severity of pain and frequency, and how it impacts people’s lives. Findings from this survey will be published online and presented at OIFE’s Topical Meeting, “Balancing Life with OI” in Stockholm, Sweden from June 9-10, 2023.
Study Announcement: Poise 1
The OI Foundation would like to bring your attention to a phase 1 study of a new medical treatment being conducted by Sanofi. Sanofi is seeking participants from the OI community.
Please take a moment to read the following message from Sanofi:
Sanofi is conducting an early phase study in adults with OI Types I and IV with an anti-TGFb antibody called SAR439459. This study is called Poise 1 and is a Phase 1 study*. This study involves a single administration of SAR439459 given intravenously (IV) into the arm, with a 6-month follow period.
At this early stage in development, Sanofi is recruiting a limited range of study participants but we do anticipate expanding enrollment criteria in future studies. Participants in the Poise 1 study are not likely to experience benefits from SAR439459, and 25% will receive a placebo, but all participants will help with the scientific understanding of OI and SAR439459 as we prepare for future long-term studies. The study also includes digital, non-invasive strategies to better understand how OI patients move and are active throughout the day.
TGFb signaling is an important part of the bone remodeling environment, playing a role in the balance of forces which remove and build new bone. In OI, signaling related to TGFb is dysregulated and Sanofi is currently the only company pursuing this specific approach to treat OI. A molecule similar to SAR439459, called fresolimumab, has been shown to increase bone mineral density in some Type IV OI patients after a single dose.
All participants will be reimbursed for travel and accommodations involved in visiting study sites during the study duration, including airfare for those who are not within driving distance of a study site. Please see the study brochure for more information about where the study sites are located and for references to the information given above.
WHAT IS A PHASE 1 STUDY?
Interested in learning more about participating in Clinical Trials? Take a look at the OI Foundation’s What You Need To Know About Clinical Trials Factsheet.
- Male or female between 18 and 65 years old, with the exception of postmenopausal women
- Confirmed diagnosis of Osteogenesis Imperfecta Types I or IV, including documented genetic mutation in the COL1A1 or COL1A2 genes. Sanofi will provide OI-specific genotyping if it is not already in the patient’s medical history.
- Have experienced ≥ 2 bone fractures since the age of 18 OR at least 1 bone fracture in the last 10 years.
If you have any questions about this study, you may email Contact-US@sanofi.com or call 800-633-1610, ext option 6 (Toll free for US & Canada).
Disclaimer: The OI Foundation is not involved in the design or management of this research, and as such, is neither endorsing nor supporting this study. The mission of the OIF is to keep the OI community informed of all relevant studies. This information is made available as a service to the OI community.
Pain and OI Project
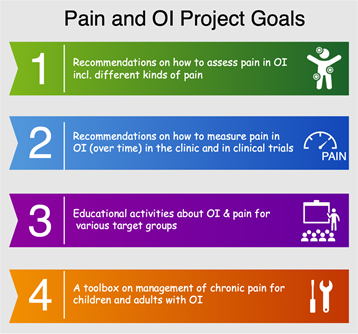
A number of recent surveys have shown that approximately 60% of individuals with OI report that they suffer from chronic pain. To increase research around pain management, the OI Foundation and Osteogenesis Imperfecta Federation Europe (OIFE) have come together to form the Pain and OI Project, an international resource group made up of a variety of professionals and representatives of OI. The project goal is to make recommendations on how to assess and measure pain, to promote educational activities about OI to target groups, and to construct a toolbox for children and adults to help manage chronic pain. Learn more about this project at https://oife.org/2022/10/28/oife-pain-oi-project/
OI Research Announcement: Use of clear aligners…

Bethesda, MD area: National Institutes of Health
Danielle Elangue danielle.elangue@nih.gov 301-771-1378
Los Angeles, CA area: UCLA
Hannah Ebbers HEbbers@mednet.ucla.edu 310.794.6420
For more information about current studies through the Brittle Bones Disorders Consortium (BBDC), visit the BBDC webpage.
Michael Geisman Fellowship Grant – Applications Now Available
Michael Geisman Fellowship Grant
The Michael Geisman Fellowship Grant program awards funding to post-doctoral trainees who are currently working on projects with clear relevance to osteogenesis imperfecta (OI), or who have projects that will enable them to develop expertise in OI research.
Applicant Requirements:
- Applicant must hold an MD, DDS, DO, or PhD, and be appointed at the level of a post-doctoral trainee, or equivalent, within an academic institution.
- Applicant should have completed their Ph.D. or clinical training within the past five (5) years.
Fellowship Guidelines:
- Michael Geisman Fellowship awards provide up to $50,000 per year. It is the intention of the OI Foundation that grant monies be used to fund actual costs related to the research being performed including Fellow salary, fringe benefits, and supplies.
- Fellowship awards are for one year; a second year of funding may be approved based upon satisfactory performance during the first year of funding.
- Research must be done under the supervision of mentor with training and experience in osteogenesis imperfecta research or research in a related field.
How to Apply:
- Complete application (pg. 2-8)
- Mentor of applicant must submit a copy of his/her NIH biosketch and a letter of recommendation on behalf of the trainee, which also confirms that the mentor will supervise the trainee’s research.
- Applications require two additional letters of recommendation from scientists or clinicians who can comment upon the applicant’s training, ability, and potential to develop expertise in OI research.
- Submit application, reference letters, and mentor biosketch as PDF documents to sconnors@oif.org NO LATER THAN November 30, 2022.
If you have any questions, please contact Stacie Connors at sconnors@oif.org.
Click here for the 2022 Michael Geisman Fellowship application.
OI Research Update: Longitudinal Study
BBDC Research Update: Longitudinal Study
On September 22, 2022, the OI Foundation was joined by Dr. Reid Sutton (Geneticist at Baylor College of Medicine, Administrative Director and Principal Investigator of the Brittle Bone Disorders Consortium, and member of the OIF Medical Advisory Council and Board of Directors) to discuss the Longitudinal Study of Osteogenesis Imperfecta. This study, also known as the Natural History Study, is the largest research project ever conducted on people with OI. Dr. Sutton discussed the design of the study, its goals, and what researchers have learned so far. Watch this session recording below.
Participate in the Longitudinal Study of OI!
The purpose of this natural history study is to perform a long-term follow-up of a large group of people with osteogenesis imperfecta (OI). Information collected will include medical history, number of broken bones, surgeries done, medications taken, ability to walk, pain, lung function and breathing, hearing, and bone mineral density. The overall goal is to improve the health and quality of life of people with OI. Click here to learn more and determine if you are eligible to participate.